Regulation and Regulatory Science
Regulation plays an essential role in evaluating the quality, safety and efficacy of medicines. It provides a framework for assessing scientific evidence, the adoption of new innovations and maintaining public trust in medicines and medical technologies that reach patients.
In the UK, the Medicines and Healthcare products Regulatory Agency (MHRA) is responsible for setting standards, evaluating new treatments, and adapting to emerging scientific advancements.
As medical research evolves, so too must regulation and regulatory practice. Innovations such as artificial intelligence, advanced therapies, and real-world data are changing the way we develop and assess new medicines.
Regulatory science helps bridge the gap between research and policy, ensuring that the latest technologies can be translated into safe and effective treatments for people and patients.

Enhancing the role of UK medicine regulation
The ABPI wants the UK to be the best place in the world to research, develop and use the medicines and vaccines of the future. UK excellence in regulation is key to the success of our sector, as it underpins the high trust and regard patients have for our products, and enables us to rapidly bring new innovations to those who can benefit most.
The ABPI published a report on ‘Enhancing the role of UK medicine regulation’. The report, made 12 recommendations to help deliver a world-class regulator that can play a leading role in the UK life sciences ecosystem, driving inward investment and facilitating patient access to innovative medicines.
Clinical research
Clinical trials are very important for finding out if a medicine works and if the benefits outweigh the risks. Every medicine used in the NHS has to be tested before it can be used in patients.
Before medicines can be researched in patients, they must undergo a rigorous series of scientific research tests. Most clinical trials start with healthy volunteers and this will be the first time the medicine will be used in people (First In Human Studies).
Phase I, II and III trials
The next step is to involve increasing numbers of patients with the disease in different phases of the clinical development programme (Phase I, II and III trials) to see if the medicine works, does it delay the disease or cure the disease, to determine the right dose and do the benefits outweigh the risks?
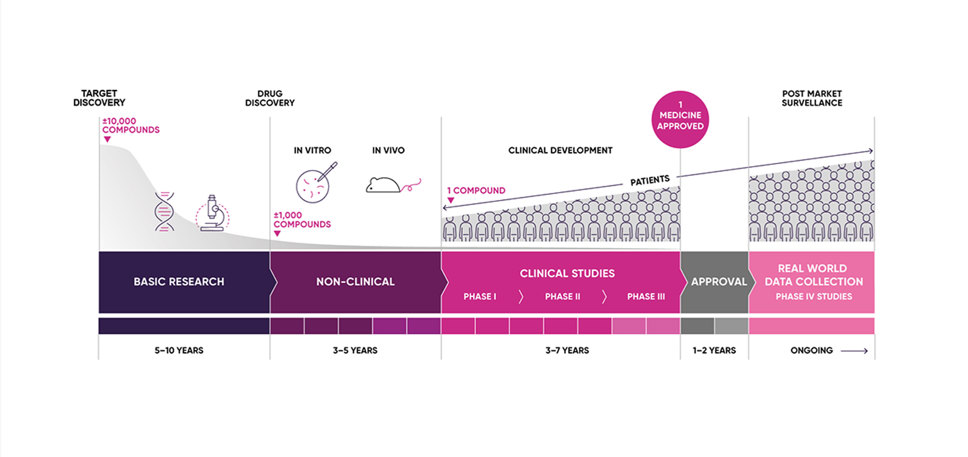
The MHRA is responsible for approving clinical trial applications in order to ensure the safety of trial participants. Clinical trials must also be conducted according to high international standards, including adhering to concepts such as Good Clinical Practice, ethics review and informed consent.
Clinical trials are key for how we develop medicines of the future, but we can’t do it without patients and we’re hugely grateful for everyone that makes them happen. Commercial trials (those that are sponsored by a pharmaceutical company) also play a large role in delivering better health and wealth for people.
A review that made recommendations on how to improve the environment for running clinical trials in the UK was commissioned by UK government ministers in 2023.
How do we approve medicines for routine use?
Clinical trials are just the beginning of a medicines journey to routine access for patients: they must be approved for use, monitored and regulated for the rest of their lifetime on the market.
A medicine’s licence application is submitted to the MHRA for review by a pharmaceutical company. The licensing process determines the quality, safety and efficacy of a medicine and if the benefits of taking the medicine outweigh the risks for a well-defined population of patients. If approved by the MHRA, the company is allowed to place the medicine on the market and this will include providing information for both the prescriber and the patient on how to use the medicine effectively.
Once a medicine is made available (subject to review processes on cost effectiveness by organisations such as NICE), the pharmaceutical company has a duty to monitor it and make sure it performs safely for as long as it’s on the market. This process if called pharmacovigilance and it helps inform regulators about previously unknown side effects – it can also help collected real world data that can be fed back to improve future research and understanding of how the medicine works.

Regulatory Science symposium
As part of the ABPI Regulatory Network’s activity, in October 2024, the ABPI hosted its first Regulatory Science Symposium, which brought together thought leaders and policymakers from across the regulatory system.
The event explored the latest trends in regulatory science and discussions on how existing national frameworks can change to better accommodate the fast pace of innovation coming from the pharmaceutical industry. The ABPI will run a second meeting later in 2025.
Centre of Excellence in Regulatory Science and Innovation (CERSI)
In January 2025, Innovate UK announced £6.2 million worth of funding for seven CERSIs to help drive advancements in healthcare. These centres focus on developing safer, faster pathways for innovative medicines and devices.
The projects picked in partnership with the MHRA, Office for Life Sciences and the Medical Research Council aim to expedite the development of life-saving treatments.
The ABPI is planning to support the work of the CERSI in order to help ensure that the UK regulatory environment remains globally competitive and can address new methods and technologies.
Related news and blogs
-

Novo Nordisk ABPI membership restored
Novo Nordisk’s two-year suspension from the Association of the British Pharmaceutical Industry (ABPI) for serious breaches of the ABPI Code of Practice has come to an end.
-

ABPI welcomes new MHRA Chief Executive Officer
Lawrence Tallon has been named as the new Chief Executive Officer of Medicines and Healthcare products Regulatory Agency (MHRA).
-
Regulatory renewal needed for UK life sciences ambitions
A new report, 'Enhancing the role of UK medicine regulation' sets out key recommendations to rebuild the UK’s world-class reputation in regulatory science, medicines’ development and licensing.
-
Getting ready for the Windsor Framework: ensuring continuity of UK medicines supply beyond 1 January 2025
The Windsor Framework, agreed between the UK and the EU in February 2023, is expected to be fully implemented on 1 January 2025.
-

How regulation can help to unlock the potential of preventative medicines
A blog post on the potential of preventative medicines from Sarah Montagne, Bayer Senior Regulatory & Policy Advisor (UKI), ABPI Regulatory Science Board Sponsored Group, Dr Dan O’Connor, ABPI Director of Regulatory Policy and Early Access and Andy Collier, Regulatory Policy Manager
-

Comparing new medicine availability across Europe
Each year, the European Pharmaceutical Industry Association (EFPIA) publishes data comparing the availability of new medicines across 36 European countries, as well as the average time it takes to make these new medicines accessible to patients. This article explains what the data shows and what it means for the UK.
-

MHRA’s new International Recognition Procedure (IRP): how does it shape up?
The MHRA has introduced a number of regulatory flexibilities for companies to consider when applying for their products to enter the UK market.
-
UK’s grand ambition for life sciences hinges on delivery
The government has moved closer to realising its life sciences vision with an ambitious and broad-ranging set of proposals to improve the clinical trials environment, but delivery and addressing acute commercial challenges remain the key to success.
Steve Hoare explains how pharmaceutical companies continue to make sure a medicine is safe, and how they use real world data to advance research and discovery.
Clinical trials are essential for testing whether new medicines are safe and effective but it really is just the beginning of the journey.
It must be monitored and regulated for their whole lifetime.
A medicine’s licence is the green light for it to be given to patients and it covers everything about how it should be used, who it's for, and what dose is appropriate.
It then needs to pass strict tests to make sure it's cost effective for the NHS.
Once a medicine is made available, the pharmaceutical company has a duty to continue to monitor it and make sure that performs safely for as long as it's on the market.
This process is called pharmacovigilance and it helps inform regulators about previously unknown side effects, but it can also help to collect additional information, real-world data that can be fed back to improve future research.
More data means a better understanding of what medicine can do often allowing it to be used to treat even more conditions or used in completely different set of patients.
The data can be fed back to progress basic science and unlock treatments for future generations.
Clinical trials are very important for finding out if a medicine works and it is safe. Every medicine used in the NHS has to be tested before it can be used.
Clinical trials start with health volunteers and this will be the first time the medicine will be used in people. The next step is then to involve patients with the disease to see if the medicine works: does it delay the disease or cure the disease, and it is safe?
Before anyone gets involved with clinical trials they’ll sit down with the team and work out exactly what’s going to happen, any of the risks and whether a clinical trial is the right thing for them.
As we get more information the trials grow, with more patients in more hospitals not just in the UK but around the world. Trials can take anywhere from weeks to years depending on the type of disease and the type of medicine that we’re studying.
This information is share with the regulator who works out whether to grant a license or not.
New data and technology mean we can do research in new ways, looking into treatments that previously seemed impossible.
The NHS is very active in research with 700,000 people taking part in research for things like cancer, heart disease and diabetes.
Clinical trials are key for how we develop medicines of the future, but we can’t do it without patients and we’re hugely grateful for everyone that makes them happen.
How do we regulate medicines?
Clinical trials are really just the beginning of a medicines journey: they must be monitored and regulated for the rest of their lifetime.
A medicine’s license is the green light for it to be given to patients and it covers everything from what it should be used for, who it should be given to and what dose is appropriate.
The next step is to past strict tests to make sure its cost effective for the NHS.
Once a medicine is made available, the pharmaceutical company has a duty to monitor it and make sure it performs safely for as long as it’s on the market.
This process if called pharmacovigilance and it helps inform regulators about previously unknown side effects – it can also help collected real world data that can be fed back to improve future research.
More data means a better understanding of what a medicine can do – often meaning we can explore using it to treat other conditions or used in other sets of patients.
It may also be fed back to unlock basic science and help progress new treatments for future generations.
Last modified: 21 March 2025
Last reviewed: 21 March 2025